by Robert W. Chandler, M.D., M.B.A. - Team 5
However, newly released internal
Pfizer documents show that this is not true. In fact, the injection
causes widespread distribution of the material in tissues and this
distribution persists for at least two days, and probably much longer.
These facts are the exact opposite of what was publicized.
A cluster of FDA-released Pfizer documents — “Final
Report: A Tissue Distribution Study of a [3H]-Labelled Lipid
Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following
Intramuscular Administration in Wistar Han Rats”[https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf], 2.4 NONCLINICAL OVERVIEW [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf], “MODULE 2.6.5. PHARMACOKINETICS TABULATED SUMMARY”
Pfizer Study 185350,” Final
Report: A Tissue Distribution Study of a [3H]-Labelled Lipid
Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following
Intramuscular Administration in Wistar Han Rat”,
is one of 21 preclinical Prizer studies involving mice, rats and rhesus
macaque non-human primates. Study No. 185350 (Sponsor Reference
ALC-NC-0552) was summarized in Pfizer’s “2.4 Nonclinical Overview” and
was separately published as a Final Report dated September 24, 2020.
Contained in that document is the following identification of the source:
Test Facility Study No. 185350 REDACTED
SPONSOR: Acuitas,
6190 Agronomy Road,
Ste. 402,
Vancouver, V6T 1Z3 Canada
Sponsor Reference No. ALC-NC-0552
This study was made up of 42 male
and 21 female Wistar Han rats. These rats were injected with 50 or 100
micrograms of BNT162b2 mRNA/LNP (lipid nanoparticle) product labelled
with a radioactive tracer material, 3H.
Then the rats were sacrificed at intervals of 0.25 hours (15 minutes); 1
hour; 2 hours; 4 hours; 8 hours; and then at 1 and 2 days.
The 100-microgram dose was associated with loss of weight and apparent toxicity in two animals. Unfortunately, the full results of the 100-microgram dose were not presented at all. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf, p. 11.]

This is very important. The 100 microgram dose was considered too
toxic to continue to use in the experiment, so the dosage was cut in
half. 100 micrograms is the amount in the Moderna injections.
The 50 microgram dose was not safe. One female rat in the 50-microgram dose exhibited piloerection and hunched posture. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf, p.19.]
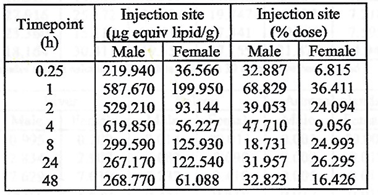
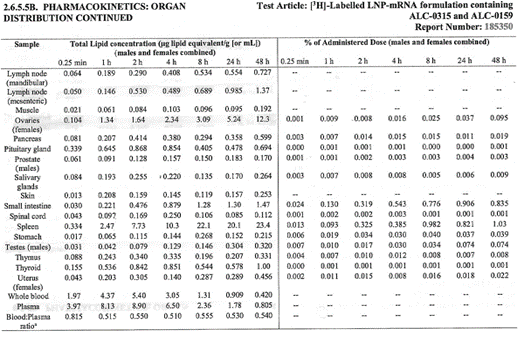
On page 20 of “Final Report: A Tissue Distribution Study of a
[3H]-Labelled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315
and ALC-0159 Following Intramuscular Administration in Wistar Han Rat,”
the authors note that widespread distribution to “most tissues” occurs
by the time of first analysis at 15 minutes after injection.
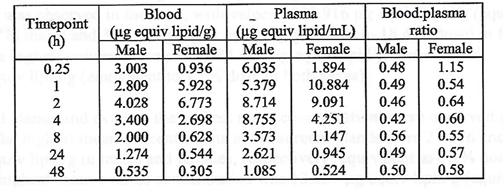
There was greater accumulation in blood when compared to plasma, and
males generally had higher concentrations than females with lower blood
to plasma ratios. No explanation for these differences was offered.
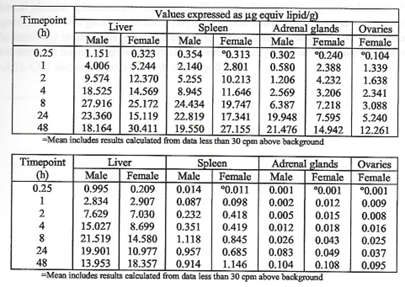
The major tissues that contained the drug concentration, aside from muscle at the injection site, were identified as being the liver, spleen, adrenal glands, and ovaries. The drug persisted in tissues throughout the duration of the study. The meaning and potential implications of the persistence in tissues was not addressed. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf, p. 21.]
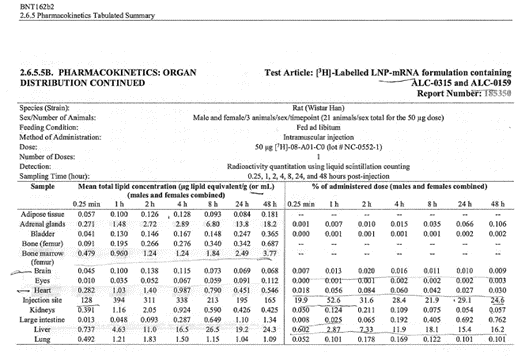
Top: highest mean concentrations. Bottom: equivalent % dose.
A separate pharmacokinetic study, “PF-07302048,” looked at the persistence of the LNP (lipid nanoparticle) transport vessel with a test mRNA inside consisting of LNP coating wrapped around Luciferase mRNA, Figure 2.4.3-1 below. [“R&D STUDY REPORT No. R-20-0072 – EXPRESSION OF LUCIFERASE-ENCODING MODRNA AFTER I.M. APPLICATION OF GMPREADY ACUITAS LIPID NANOPARTICLE FORMULATION”, https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_R-20-0072.pdf.]
The object of this study was to follow the LNP vessel in plasma and liver, and then measure transcription of mRNA inside target organs to validate the delivery model using the bioluminescent properties of Luciferase to identify transcription of the mRNA in target tissues. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_R-20-0072.pdf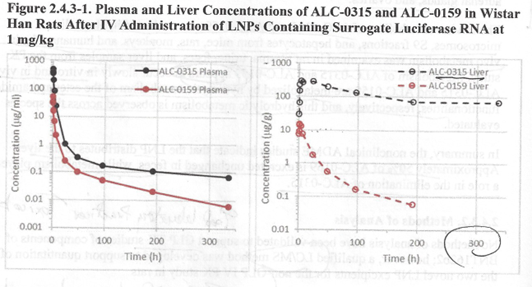
With respect to degradation of the mRNA component, we learn from “2.4 Nonclinical Overview” that Pfizer/Acuitas did not study at all the degradation of the synthetic mRNA in BNT162b2. Similarly, there was no analysis by Pfizer of protein products from BNT162b2 provided. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p.20.]

The final chart on page 19 contrasts ovaries vs testes with approximately 38 times more drug concentration of drug in ovaries. More work is needed to see if there is a connection between menstrual cycle changes as have been reported by Lee, et. al. and elsewhere.
Similarly, although the data is incomplete with known outcome in only 27 of 270 pregnancy cases as reported in Pfizer document 5.3.6, the first three months of Adverse Events reporting following widespread release after an Emergency Use Authorization was given by the FDA. What data does exist is disconcerting.
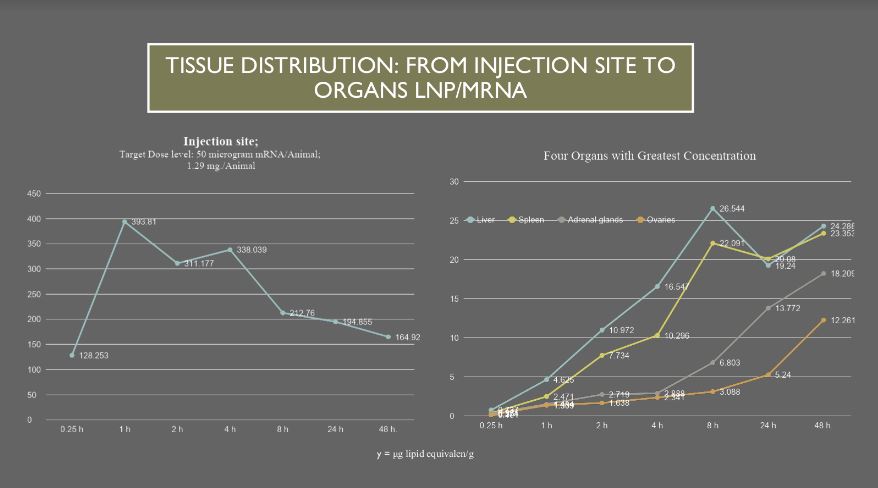
Several serious questions are raised by these results:
- How long does the BNT162b2 mRNA persist in human tissues? Where does it go in the host cell? How long does it persist inside the cell? What proteins does it produce, and for how long?
- Is there any possibility that the BNT162b2 mRNA can be transcribed into DNA, then incorporate into the host genome? If this happens what are the implications?
- What are the toxicities from the lipid nanoparticle coating?
- Was Pfizer obligated to answer these questions prior to human testing?
- Doesn’t proper informed consent require answers to these questions?
Fortunately, answers to these important questions are beginning to appear:
1a. Duration of mRNA in tissues:
In a July 19, 2022, article, the essayist Joomi reviews the topic of how long BNT162 b2 containing mRNA stabilized by a synthetic nucleotide 1N-methyl pseudouridine persists in human tissues. [https://joomi.substack.com/p/were-still-being-misled-about-how?r=chkp3&s=r&utm_campaign=post&utm_medium=web]
A January 2022 human lymph node biopsy study from Stanford University found that the mRNA from both Pfizer and Moderna persists for at least two months, which was the duration of the study. [https://www.cell.com/action/showPdf?pii=S0092-8674%2822%2900076-9]
1b. Proteins produced from BNT162b2 mRNA:
Spike protein is produced after the mRNA is transcribed, and has been found in vivo for at least four months after inoculation. [https://joomi.substack.com/p/were-still-being-misled-about-how?r=chkp3&s=r&utm_campaign=post&utm_medium=web]
Proteins transcribed from the mRNA have not been completely characterized yet. SARS-CoV-2-like Spike protein has been identified as long as four months after inoculation with LNP/mRNA in human exosomes. Toxicity of Spike protein has been described and is reviewed in the essay “We’re still being misled about how long the mRNA vaccines last in the body.” [https://joomi.substack.com/p/were-still-being-misled-about-how?r=chkp3&s=r&utm_campaign=post&utm_medium=web]
2. What is the fate of BNT162b2 mRNA?
We were informed that “RNA is required for protein synthesis, does not integrate into the genome, is transiently expressed, and is metabolized and is eliminated by the body’s natural mechanisms and, therefore is considered safe.” [Alberer, M. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomized, prospective, first-in-human phase 1 clinical trial. Lancet 90, 1511-1520 (2017).] [Sahin, U. e al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222-226 (2017).]
However, Alden, et. al., reporting in Current Issues in Molecular Biology 2022, 44, 1115-1126, found BNT162b2 mRNA is reverse transcribed into host DNA beginning six hours after contact with BNT162b2:
“In the BNT162b2 toxicity report, no genotoxicity nor carcinogenicity studies have been provided. Our study shows that BNT162b2 can be reverse transcribed to DNA in liver cell line Huh7, and this may give rise to the concern if BNT162b2-derived DNA may be integrated into the host genome and affect the integrity of genomic DNA, which may potentially mediate genotoxic side effects. At this stage, we do not know if DNA reverse transcribed from BNT162b2 is integrated into the cell genome. Further studies are needed to demonstrate the effect of BNT162b2 on genomic integrity, including whole genome sequencing of cells exposed to BNT162b2, as well as tissues from human subjects who received BNT162b2 vaccination.” [https://www.mdpi.com/1467-3045/44/3/73/htmThis study did not identify DNA transcribed from BNT162b2 mRNA in the host genome following transcription.
However, Zhang et. al., working at Massachusetts Institute of Technology, demonstrated fragments of SARS-CoV-2 mRNA integrated in host DNA in “Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues,” published in 2021 in PNAS, vol. 118, no. 21:
“We show here that SARS-CoV-2 RNA can be reverse-transcribed and integrated into the genome of the infected cell and be expressed as chimeric transcripts fusing viral with cellular sequences. Importantly, such chimeric transcripts are detected in patient-derived tissues.” [https://www.pnas.org/doi/10.1073/pnas.2105968118]
So, scientists are getting close to knowing whether BNT162b2, with its synthetic mRNA, is translated into host DNA and is now a permanent part of human genetic material. If so, the next step is to determine what the implications are.
3. What are the toxicities from the lipid nanoparticle coating?
More research is required to understand the implications of LNP concentration in various organ tissues. It is thought that the PEG component (the polyethylene glycol that coats the LNP) is responsible for anaphylaxis, an often rapid-onset major physiologic event that requires emergency treatment.
4. Was Pfizer obligated to answer these questions prior to human testing?
5. Doesn’t proper informed consent require answers to these questions?
The answers to questions 4 and 5 are “yes,” and the reasons should be obvious now. Basic information about functioning of this mRNA product, BNT162b2, was not known at the time of mass inoculation; and, therefore, a proper risk, benefits and complications discussion was compromised by lack of information. Informed consent is not possible in such a situation.
In conclusion, many negatively consequential shortcuts were made in the development of BNT162b2.
Many omissions in basic research evaluation of BNT162b2 were kept hidden, and there was outright misinformation regarding some of the work that was done.
Assumptions rather than actual research to determine where BNT162b2 goes, what it does, and how long it lasts were made that proved to be false and constitute intentional mis/dis/mal information. We were told that the prodrug, BNT162b2, consisting of a lipid nanoparticle coating of synthetic messenger ribonucleic acid (modRNA), would be deposited in muscle tissue at the injection site and would be migrate to local lymphatics prior to rapid degradation producing Spike antigens for a limited period of time that would produce a desired immune response.
However, Pfizer in its very early Phase 1 trial with mice, rats, and rhesus non-human primates learned that the LNP/mRNA is rapidly disseminated throughout the body and remained in tissues for as long as it was studied, 48 hours for BNT162b2 and 12.5 days for the LNP/Luciferase mRNA test product.
No effort was expended to determine what proteins are produced by the modRNA, what their physiological actions are and how long they are produced as well as what toxicities and adverse events might be anticipated with widespread usage of the LNP/mRNA prodrug.
FOIA requests for internal documents from federal health care agencies, independent review board members, approximately 140 clinical investigators and Pfizer personnel should be made.
Billions of doses were administered to billions of p
eople. The scale of this potentially massive medical misstep is large.
Ten months to develop novel gene therapy for a novel virus is well short of the five to 10 years usually required to develop, test and refine such a product. After billions of doses have been given to children and adults around the world, possibly altering the course of human evolution, the public is now seeing the unfortunate consequences of cutting corners.
This Report was written exclusively for DailyClout by the Members of the War Room / DailyClout Pfizer Documents Research Volunteers.
It should not be copied or republished without permission from DailyClout or a full credit and link to DailyClout.io